PPM → pH Calculator
Estimate pH for dilute strong acid or base solutions based on concentration in PPM.
Extremely high or low concentrations may yield approximate results.
Water PPM to pH Calculator – Determine pH from TDS & Chemical Levels
Estimate water pH using PPM/TDS data. Useful for pool balancing, chlorination, filtration, and water treatment diagnostics. Includes typical pH vs PPM correlations.
Welcome to our comprehensive guide on converting Parts Per Million (PPM) to pH. While these two measurements are fundamental in chemistry, especially in fields like hydroponics, water treatment, and aquarium maintenance, they measure entirely different things.
A common point of confusion is how to relate them. Our PPM to pH Calculator is designed to bridge that gap, but understanding the science behind it is key to using it effectively.
How to Use the PPM to pH Calculator
Our tool is split into two main sections to make your conversions seamless, whether you’re starting with a PPM value or a pH reading.
Converting PPM to pH
This section is perfect when you know the concentration of a specific chemical in your water (in PPM) and need to find out its impact on the acidity or alkalinity (the pH).
- Enter PPM (mg/L): In the first field, type in the concentration of your substance. For dilute water solutions, PPM is equivalent to milligrams per liter (mg/L).
- Select Substance: This is the most crucial step. From the dropdown menu, choose the acid or base that you’re measuring. The chemical’s unique properties (specifically its molar mass) are essential for an accurate conversion.
- Click “Convert to pH”: The calculator will instantly perform the conversion, and your resulting pH value will appear below the button.
Converting pH to PPM
Use this section when you have a pH reading and want to determine what concentration of a specific acid or base is required to achieve it.
- Enter pH: In the top field, input the pH value of your solution. The acceptable range is from 0 to 14.
- Select Substance: Just as before, you must select the correct substance from the dropdown list to ensure the calculation is accurate for that specific chemical.
- Click “Convert to PPM”: The tool will calculate the required concentration, and the result in PPM (mg/L) will be displayed in the box below.
Formula & Conversion Explanation
You might wonder why you can’t just plug PPM into a simple formula to get pH. The reason is that they measure fundamentally different properties of a solution. PPM is a measure of mass concentration, while pH is a measure of ion activity. The conversion is a multi-step process that involves bridging this gap.
What is PPM (Parts Per Million)?
PPM is a straightforward way to describe the concentration of something in a larger solution. It tells you how many “parts” of a substance you have for every one million parts of the total solution.
For water-based solutions, this is made very simple: 1 PPM is equivalent to 1 milligram of a substance dissolved in 1 liter of water (1 mg/L).
Think of it like this: If you had a million grains of sand and one of them was red, the concentration of red sand would be 1 PPM. It’s a convenient unit for measuring very low concentrations of solutes, like minerals in drinking water, nutrients in a hydroponic setup, or chlorine in a swimming pool.
What is pH?
The pH scale measures how acidic or alkaline a water-based solution is. The scale typically runs from 0 to 14.
- A pH of 7 is neutral (like pure water).
- A pH less than 7 is acidic (like lemon juice or vinegar).
- A pH greater than 7 is alkaline or basic (like baking soda or bleach).
What the pH scale is actually measuring is the concentration of hydrogen ions (H⁺). Here’s the important part: it’s a logarithmic scale. This means that for each one-point decrease on the scale, the acidity increases by a factor of 10. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and one hundred times more acidic than a solution with a pH of 6.
PPMCalculator.com provides fast, accurate concentration and conversion tools to support chemistry, engineering, agriculture, and laboratory workflows.
The Conversion Process: From Mass to Ions
Because there’s no direct formula, we have to go through a few steps. The key that unlocks the conversion is a concept from chemistry called molarity.
- PPM (mg/L) = Mass concentration
- Molarity (mol/L) = Molar concentration (number of molecules)
- pH = Based on the concentration of ions
Here’s a breakdown of the chemistry our calculator performs for you.
The Step-by-Step Journey from PPM to pH
Let’s say you have 150 PPM of Hydrochloric Acid (HCl).
- Convert PPM to Grams per Liter (g/L): Since 1 g = 1000 mg, we simply divide the PPM by 1000.
150 mg/L / 1000 = 0.15 g/L
- Convert g/L to Molarity (mol/L): This is where the specific substance matters. We need its molar mass (the weight of one mole of the substance). The molar mass of HCl is 36.46 g/mol.
0.15 g/L / 36.46 g/mol = 0.0041 mol/L
- Determine Hydrogen Ion [H⁺] Concentration: We are using strong acids and bases, which fully dissociate (break apart) in water. HCl releases one hydrogen ion per molecule.
[H⁺] = 0.0041 mol/L
- Calculate pH: The formula for pH is
-log₁₀[H⁺].pH = -log₁₀(0.0041) ≈ 2.39
So, a 150 PPM solution of HCl has a pH of approximately 2.39.
Applications & Use Cases of PPM to pH Calc
This conversion is critical in many industries and hobbies where water chemistry must be precisely controlled.
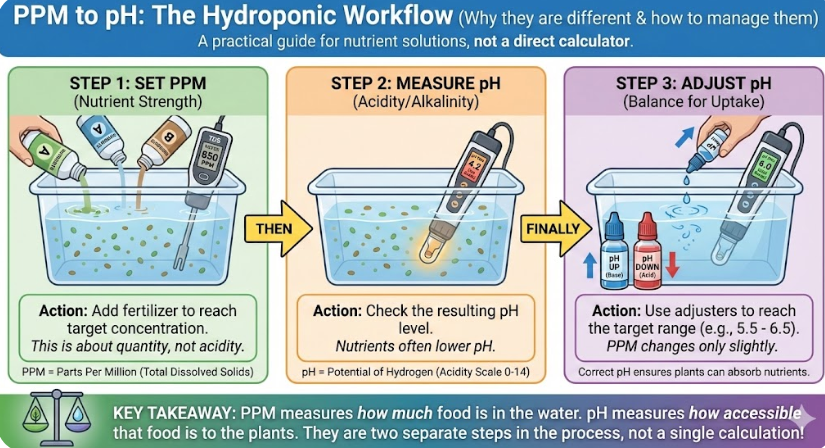
Hydroponics and Agriculture
In hydroponics, plants are grown without soil, receiving their nutrients from a water solution. Growers measure the strength of their nutrient solution in PPM. However, plants can only absorb these nutrients within a specific pH range. If the pH is too high or too low, the nutrients are “locked out” and unavailable to the plant, even if the PPM reading is perfect. Growers constantly use pH adjusters (acids or bases) and must understand how their PPM will affect the final pH of the solution.
Aquariums and Aquaculture
Maintaining a stable pH is vital for the health of fish and other aquatic life. Different species thrive in different pH ranges. The substances dissolved in the water—from fish waste breaking down into ammonia/nitrates (measured in PPM) to added water conditioners—all influence the pH. Understanding this relationship helps aquarists maintain a stable and healthy environment.
Water Treatment
Municipal water treatment facilities must ensure our drinking water is safe. They add chemicals like chlorine (measured in PPM) to disinfect the water. However, the pH of the water must also be controlled. It needs to be kept close to neutral (7.0) to prevent pipe corrosion and ensure the chlorine is effective.
Swimming Pool and Spa Maintenance
Just like in water treatment, the concentration of sanitizers like chlorine or bromine is measured in PPM. The pH of the pool water needs to be kept in a narrow range (typically 7.2 to 7.8). If the pH is off, it can cause skin and eye irritation for swimmers and reduce the effectiveness of the chlorine.
Related Calculators and Resources
- mg/L to PPM Calculator
- PPM to EC (Conductivity) Calculator
- PPM Conversion Table
- PPM vs TDS vs EC
- How to Calculate PPM Step-by-Step
- PPM Calculation Examples
- PPM Accuracy Calculator
- PPM for Water Testing
- Pool pH Acid Demand Calculator
Frequently Asked Questions (FAQs)
1. Why can’t you convert PPM to pH directly?
You can’t convert them directly because they measure different things. PPM measures the concentration of a substance by mass, while pH measures the activity of hydrogen ions on a logarithmic scale. The conversion depends on the substance’s molar mass and how many hydrogen (H⁺) or hydroxide (OH⁻) ions it releases in water.
2. Is this calculator accurate for any substance?
No. This calculator is designed for the strong acids and bases listed in the dropdown. These chemicals are predictable because they dissociate completely in water. Weak acids and bases (like vinegar or baking soda) only partially release their ions, which requires a much more complex calculation involving an equilibrium constant (Ka or Kb). Using this tool for a substance not on the list will give you an incorrect result.
3. What is the difference between PPM and TDS?
TDS stands for Total Dissolved Solids. A TDS meter doesn’t actually measure the solids; it measures the electrical conductivity (EC) of the water and estimates the TDS based on that reading. It gives you a general idea of how much “stuff” is dissolved in your water but doesn’t tell you what that stuff is. PPM, in the context of our calculator, refers to the known concentration of one specific, identified substance.
4. Does temperature affect the PPM to pH calculation?
Yes, technically. The pH of a solution is temperature-dependent. The standard pH scale where 7 is neutral is defined at 25°C (77°F). Our calculator, like most standard tools, assumes this temperature. While the effect is minor for most general use cases, it can be significant in high-precision scientific and industrial settings.
5. Why is the pH scale logarithmic?
The concentration of hydrogen ions in solutions can vary enormously—over many orders of magnitude. Using a logarithmic scale compresses this vast range into a manageable 0-14 number system. It makes it much easier to express and work with these values. Without it, you’d be dealing with numbers like 0.0000001 moles/liter instead of simply saying “pH 7.”
6. Is a high PPM reading always a bad thing?
Not at all! It completely depends on what the substance is. A high PPM of a beneficial nutrient in a hydroponic solution is good. A high PPM of dissolved minerals (like calcium and magnesium) might just mean you have “hard water,” which is generally safe. However, even a very low PPM of a contaminant like lead or mercury is extremely dangerous. PPM is just a measurement; the context is everything.
