Easily convert 50 ppm to percent (0.005%) with a simple formula and real-world examples. Learn what ppm means, how to calculate it, and where it’s used in labs, water testing, and fertilizers on PPM Calculator.
What 50 PPM Means
If you’ve ever seen a measurement labeled 50 ppm, you’re looking at 50 parts per million.
This unit is common in chemistry, water analysis, food safety, and agriculture — anywhere precise concentration measurements matter.
It simply means 50 parts of a substance exist in one million parts of total mixture.
Because the numbers are so small, ppm is more practical than using percentages — until you need to translate it into a percent value.
How to Convert 50 PPM to Percent
The conversion is simple and always follows the same rule:
1 ppm=0.0001%
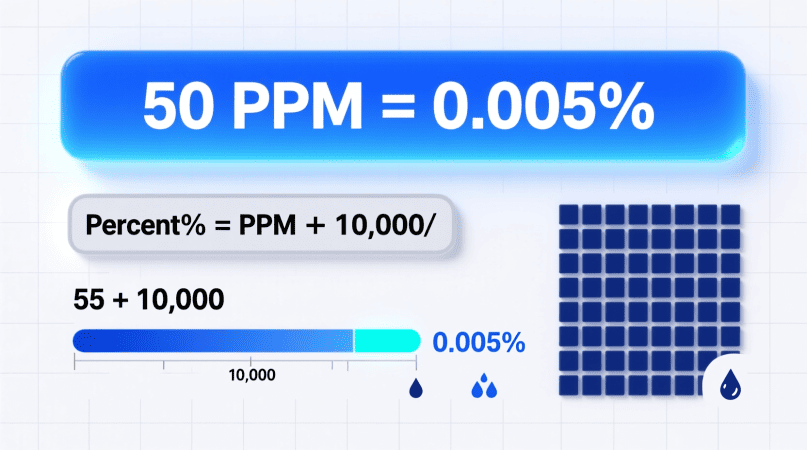
So, 50 ppm=50×0.0001=0.005%
✅ Answer: 50 ppm = 0.005%
That means a solution with 50 ppm of a substance contains 0.005% of that substance by mass or volume.
If you’d like to check your numbers instantly, you can use the PPM to Percent Calculator — a reliable tool for quick and accurate conversions.
Formula for Converting PPM to Percent
The universal formula is:
Percent = PPM/10,000
Substituting 50 for ppm: 50÷10,000=0.005%
This equation works in both directions. To find ppm from percent, multiply by 10,000.
For instance, 0.005% × 10,000 = 50 ppm — confirming the relationship is exact.
If you deal with larger or smaller concentrations often, tools like the PPM Solution Calculator and the PPM Conversion Table can help simplify your workflow.
Where 50 PPM Conversion Is Commonly Used
- In water testing: 50 ppm of total dissolved solids equals 0.005% concentration. You can compare similar values using the TDS to PPM Calculator.
- In pool maintenance: Chlorine levels are usually around 1–3 ppm. A reading of 50 ppm (0.005%) would be too high — verify proper dosing with the Chlorine PPM Calculator.
- In agriculture: Fertilizer and nutrient solutions might be mixed to achieve 50 ppm of specific elements like magnesium or iron. Try the Nutrient PPM Calculator for precision.
- In manufacturing or lab calibration: 50 ppm impurity equals 0.005% — a small, but measurable, concentration that could affect product quality. Learn how labs measure this in the How to Calculate PPM in Chemistry guide.
Why Understanding PPM-to-Percent Conversion Matters
Even though 50 ppm seems like a tiny number, in chemistry or environmental monitoring, it can have real implications. For instance:
- 50 ppm of a disinfectant might be safe for cleaning but unsafe for drinking.
- 50 ppm of a trace metal in fertilizer can improve plant health.
- 50 ppm of impurities in production might trigger a quality control flag.
Knowing how to interpret ppm as a percentage (0.005%) helps you compare and communicate these concentrations in more intuitive terms.
Avoiding Common Mistakes
- Don’t confuse ppm with mg/L in gases or solids. They’re equivalent only in dilute aqueous solutions.
- Always divide by 10,000, not 1000.
- Keep results to at least four decimal places when reporting ppm conversions.
Summary
50 ppm equals 0.005%.
That’s fifty parts of a substance in one million parts of a solution — a concentration small enough for lab analysis but significant in fields like agriculture and water safety.
For precise conversions and practical calculators, visit PPM Calculator — your trusted source for ppm, percent, and concentration tools.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
