If you’ve ever worked with chemical solutions or lab measurements, you’ve likely heard of PPM — short for parts per million. But how do you actually calculate ppm from concentration?
This guide breaks down what ppm means, how it’s related to concentration, and how you can calculate it accurately in chemistry, water testing, or environmental studies.
What Does PPM Mean?
PPM (parts per million) expresses how many parts of a solute are present in one million parts of a total solution.
It’s typically used for very low concentrations — like measuring dissolved solids, trace metals, or pollutants.
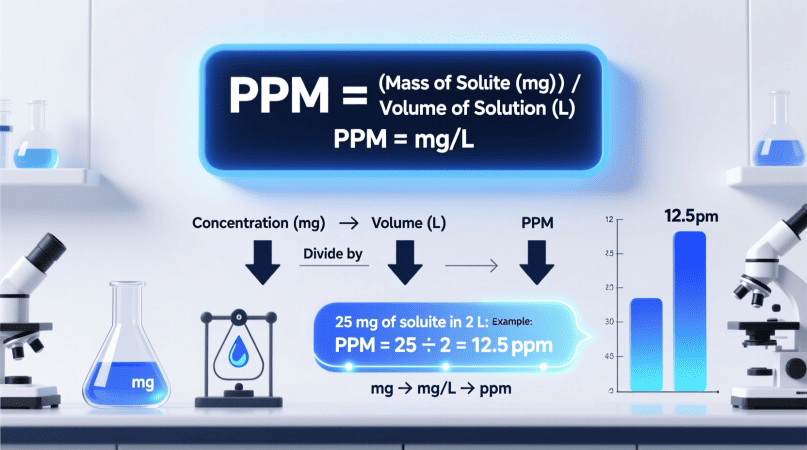
In chemistry terms: 1ppm=1mg of solute per liter of solution (mg/L)
That’s equivalent to 1 milligram of solute dissolved in one liter of water.
The Basic Formula to Calculate PPM from Concentration
When you know the concentration of a substance (in mg/L, g/L, or mol/L), you can convert it to ppm using one of the following formulas:
1. When concentration is in mg/L
PPM=Concentration (mg/L)
Because 1 mg/L equals 1 ppm for dilute aqueous solutions, this is the simplest conversion.
2. When concentration is in g/L
PPM=Concentration (g/L)×1000
(1 g/L = 1000 mg/L = 1000 ppm)
3. When concentration is in mol/L (molarity)
PPM=Molarity (mol/L)×Molar Mass (g/mol)×1000
This formula is used when working with chemical compounds in molar solutions.
You can verify your conversion instantly with the PPM Solution Calculator.
Example 1: Calculate PPM from mg/L
If the concentration of calcium in a water sample is 40 mg/L, then:
PPM= 40
✅ The solution has 40 ppm calcium.
This relationship is especially useful in environmental chemistry, where ppm and mg/L are often used interchangeably for dilute water solutions.
Example 2: Calculate PPM from g/L
Suppose a solution has 0.005 g of NaCl per liter.
Convert grams to milligrams: 0.005g/L×1000=5mg/L
Now apply the ppm formula:
PPM= 5
✅ The NaCl concentration is 5 ppm.
If you’d rather skip the math, our PPM to Percent Calculator automatically performs these conversions.
Example 3: Calculate PPM from Molarity
Let’s say you have a 0.001 M (mol/L) solution of sodium chloride (NaCl).
NaCl’s molar mass = 58.44 g/mol.
PPM=0.001×58.44×1000=58.44
✅ The 0.001 M NaCl solution is 58.44 ppm.
You can confirm this value using the Molarity Calculator.
Understanding Concentration Units
Here’s a quick reference to relate ppm with other common concentration units in chemistry:
| Unit | Equivalent to | Conversion to PPM |
|---|---|---|
| mg/L | 1 ppm | × 1 |
| g/L | 1000 ppm | × 1000 |
| % (percent) | 10,000 ppm | × 10,000 |
| mol/L | depends on molar mass | molarity × molar mass × 1000 |
These conversions make ppm a universal measure of concentration across different experiments. For more on common errors during conversion, see PPM Calculation Mistakes.
Why Chemists Use PPM for Concentration
PPM simplifies comparisons between substances in very dilute solutions.
Instead of writing tiny decimal percentages (like 0.0001 %), ppm expresses them cleanly — for example, 0.0001 % = 1 ppm.
It’s the preferred unit for:
- Water quality analysis (chlorine, iron, fluoride)
- Air monitoring (CO₂, ozone, NO₂ levels)
- Laboratory testing (trace chemicals in solutions)
- Agricultural chemistry (nutrient or fertilizer dosing)
For agricultural calculations, you can explore our Nutrient PPM Calculator.
Common Conversion Errors
- Wrong density assumption – ppm ≈ mg/L only when solution density ≈ 1 g/mL (typical for water).
- Molar mass confusion – always use accurate molecular weight for molar-to-ppm conversions.
- Improper rounding – ppm calculations often require precision to at least two decimal places.
- Temperature variations – density changes at high temperatures can slightly alter ppm readings.
To maintain consistent accuracy, check your inputs with the PPM Accuracy Calculator.
Frequently Asked Questions
1. How do you calculate ppm from concentration percent?
Multiply the percentage by 10,000. For example, 0.02 % × 10,000 = 200 ppm.
2. Is 1 ppm equal to 1 mg/L?
Yes, for water and dilute aqueous solutions.
3. How do you find ppm from molarity?
Use: ppm = molarity × molar mass × 1000.
4. Why use ppm instead of molarity?
PPM provides a simpler way to express small concentrations, while molarity is more useful for reaction stoichiometry.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
