If you’ve ever asked, “How do you calculate ppm of a solution?”, you’re already thinking like a chemist. PPM, short for parts per million, is one of the simplest yet most precise ways to describe how much of one substance is dissolved in another.
Understanding ppm is essential for chemistry, environmental science, food testing, and water treatment. Let’s go step by step and learn how to calculate ppm, why it matters, and how to apply it in real-world lab or field situations.
What Does PPM Mean in a Solution?
PPM (parts per million) measures how many parts of solute are present in one million parts of a solution.
Think of it as a ratio — it expresses very small concentrations that would otherwise be difficult to describe with percentages.
For example:
- 1 ppm = 1 milligram of solute per liter of water (1 mg/L).
- 10 ppm = 10 mg of solute per liter of solution.
So if you dissolve 5 mg of salt in 1 liter of water, that solution’s concentration is 5 ppm.
If you’re new to the concept, visit our PPM Glossary — it explains related terms like TDS, EC, mg/L, and ppb, which are often used alongside ppm.
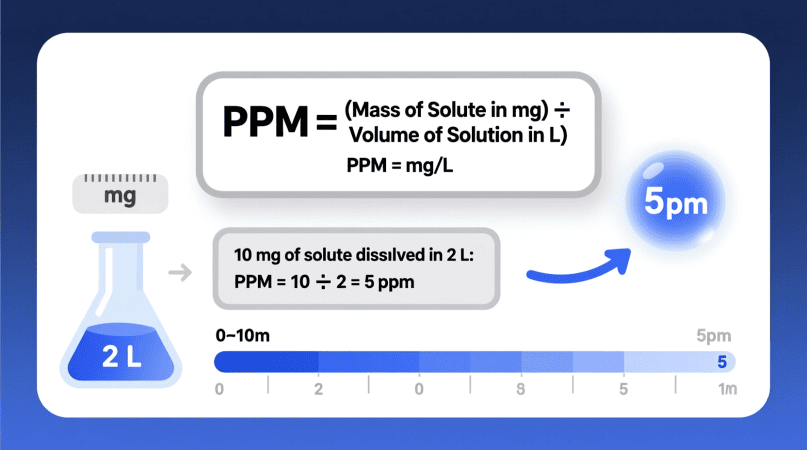
The Formula to Calculate PPM of a Solution
The simplest formula for ppm is:
PPM = {Mass of Solute (mg)} / {Mass or Volume of Solution (L or kg)}
This formula works perfectly when dealing with aqueous solutions, since 1 liter of water weighs approximately 1 kilogram.
To make it practical:
PPM = mg of Solute / L of Solution
That means:
- 1 mg of solute in 1 L = 1 ppm
- 10 mg of solute in 2 L = 5 ppm
- 100 mg of solute in 10 L = 10 ppm
You can find alternate formulas for solids, liquids, and gases in our PPM Formula Variations.
Example: How to Calculate PPM Step by Step
Let’s take a real chemistry example.
Question:
What is the ppm of a sodium chloride (NaCl) solution if 25 mg of salt is dissolved in 2 liters of water?
Step 1: Identify the data.
- Solute (NaCl): 25 mg
- Solution volume: 2 L
Step 2: Apply the formula.
PPM = 25/2= 12.5
Answer:
The solution concentration is 12.5 ppm.
If you prefer not to calculate manually, try our PPM Solution Calculator.
It instantly converts mass and volume into ppm and saves time for lab technicians or students checking their results.
Converting PPM into Other Units
Once you have ppm, you can convert it into related concentration units like percent, molarity, or mg/L depending on your experiment.
1. Converting PPM to Percent
Use this formula:
Percen = PPM/10,000
So, 50 ppm = 0.005%.
For faster results, use our PPM to Percent Calculator.
2. Converting PPM to Molarity
When you know the molecular weight (molar mass) of your solute:
Molarity = PPM/Molar Mass* 1000
This conversion is helpful for chemical reaction calculations. You can check or cross-verify values with our Molarity Calculator.
3. PPM and mg/L
In most water-based solutions:
1 ppm= 1 mg/L
They’re essentially interchangeable when dealing with dilute aqueous solutions.
Why PPM Calculations Are Important
PPM is the preferred way to measure small quantities because it gives a clear picture of concentration without switching to decimals or scientific notation.
Here are common areas where ppm is critical:
- Water treatment: Measuring chlorine, fluoride, or mineral content.
- Environmental testing: Checking for contaminants or metals in water and soil.
- Food chemistry: Monitoring additives or trace residues.
- Pharmaceuticals: Ensuring correct solution dosages.
For environmental testing examples, read our guide on Environmental Monitoring Using PPM.
Common Mistakes When Calculating PPM
Even small errors can create inaccurate ppm results. Here are mistakes to avoid:
- Using the wrong units.
Always convert grams to milligrams (1 g = 1000 mg) before dividing. - Assuming all solutions have the same density.
The shortcut 1 L = 1 kg only works for water-based solutions. - Forgetting to factor temperature.
Density and solubility can shift slightly with temperature changes. - Rounding too early.
Keep full decimal precision until the final step.
If you’ve run into calculation problems, see our troubleshooting guide on PPM Calculation Mistakes.
Frequently Asked Questions
1. What does 10 ppm mean in a solution?
It means there are 10 parts of solute for every million parts of solution — or 10 mg per liter if the solvent is water.
2. How do you calculate ppm from percent?
Multiply the percentage by 10,000. For example, 0.002% × 10,000 = 20 ppm.
3. How do you prepare a 1 ppm solution?
Dissolve 1 mg of your solute in enough solvent to make 1 liter of solution. Adjust quantities proportionally for larger batches.
4. Is ppm the same as mg/L?
For water and dilute solutions, yes. 1 mg/L equals 1 ppm in most chemistry and environmental calculations.
Key Takeaways:
- Formula: ppm = mg solute ÷ L solution.
- 1 ppm = 1 mg/L for water-based solutions.
- Always check units and keep temperature consistent.
- Use calculators for fast, accurate results.
Understanding ppm gives you control and confidence in chemical measurements, especially when working with precise lab data or environmental samples.
For accurate, ready-to-use tools, explore our PPM Solution Calculator and other chemistry converters on ppmcalculator.com.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
