If you’re working in chemistry, water treatment, or food safety, you’ve probably encountered instructions to “make a 200 ppm solution.”
But what does that actually mean? And how can you mix it correctly — whether it’s for chlorine, hydrogen peroxide, or fertilizer use?
This guide explains how to calculate and prepare a 200 ppm solution safely and accurately, using simple examples and formulas anyone can follow.
What Does 200 PPM Mean?
PPM (parts per million) expresses the concentration of a solute (the substance you’re adding) in a solution.
A 200 ppm solution means that for every 1 million parts of total solution, there are 200 parts of solute.
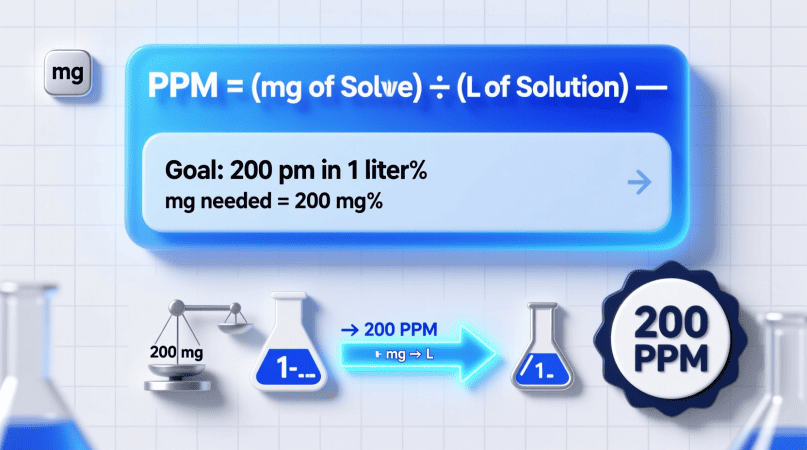
1ppm=1mg of solute per liter of solution (mg/L)
so, 200ppm=200mg of solute per liter of solution
That’s the equivalent of dissolving 0.2 grams of solute in 1 liter of water.
If you’re new to ppm calculations, see our PPM Glossary for related terms like mg/L, molarity, and EC.
Formula to Calculate a 200 PPM Solution
The standard ppm formula is: PPM=Mass of Solute (mg) / Mass or Volume of Solution (L or kg)
To find out how much solute you need, rearrange it: Mass (mg)=PPM×Volume (L)
Example:
To make 1 liter of a 200 ppm solution: Mass=200×1=200mg
That means you’ll dissolve 200 milligrams (0.2 g) of solute into 1 liter of water.
If you’re making larger batches:
- 5 L solution: 200 × 5 = 1000 mg = 1 g
- 10 L solution: 200 × 10 = 2000 mg = 2 g
How to Make 200 PPM Chlorine Solution (Example)
Chlorine solutions are commonly prepared for cleaning and sanitizing.
The goal is to mix bleach (sodium hypochlorite) with water until you reach a 200 ppm concentration of available chlorine.
Here’s how to do it safely:
Step 1: Know the strength of your bleach
Most household bleach is 5% sodium hypochlorite, which equals about 50,000 ppm.
Step 2: Use the dilution formula
C1×V1=C2×V2
Where:
- (C_1) = starting concentration (e.g., 50,000 ppm)
- (V_1) = volume of bleach to use
- (C_2) = desired concentration (200 ppm)
- (V_2) = final volume of solution (e.g., 1 L)
Plug in the numbers:
50,000×V1=200×1000
V1 = 200,000 / 50,000 = 4 mL
So, to make 1 liter of 200 ppm chlorine solution, mix 4 mL of 5% bleach with 996 mL of water.
For accuracy, try our Bleach PPM Calculator.
How to Make 200 PPM Hydrogen Peroxide Solution
Hydrogen peroxide (H₂O₂) is another sanitizer commonly diluted for safe use.
- Start with 3% hydrogen peroxide (≈ 30,000 ppm).
- Use the same dilution equation: C1×V1=C2×V2
30,000×V1=200×1000
V1=6.7mL
So, you need 6.7 mL of 3% hydrogen peroxide per 1 liter of water.
To simplify this calculation, use our Hydrogen Peroxide PPM Calculator.
Example: 200 PPM Nutrient or Fertilizer Solution
In hydroponics and agriculture, a 200 ppm nutrient mix helps maintain balanced plant growth.
The process is similar — you’ll just be dissolving a solid fertilizer or nutrient powder instead of a chemical sanitizer.
If you want 200 ppm nitrogen using a fertilizer like calcium nitrate (which contains about 15% nitrogen):
- Each gram provides 150 mg of nitrogen.
- To reach 200 ppm (200 mg/L), divide:
200/150 = 1.33 g per liter - Dissolve 1.33 grams of calcium nitrate per liter of water.
You can fine-tune agricultural and hydroponic mixes with our Nutrient PPM Calculator.
Safety Tips When Preparing 200 PPM Solutions
- Use protective gear: Gloves, goggles, and a clean workspace.
- Use distilled water: Tap water can alter ppm results due to existing minerals.
- Label all containers: Include concentration, date, and contents.
- Avoid mixing chemicals: Never mix chlorine with ammonia or acids.
- Check your ppm levels: Use test strips or a digital meter to confirm the target concentration.
If you’re troubleshooting inconsistent results, review our PPM Calculation Mistakes Guide.
Frequently Asked Questions
1. Why use 200 ppm?
It’s strong enough for effective sanitation or nutrient delivery without being corrosive or toxic.
2. How do I test my 200 ppm solution?
Use a ppm meter or color test strip for chlorine or nutrient concentration.
3. Can I store a 200 ppm solution?
Yes, but for chlorine and hydrogen peroxide, potency drops after 24–48 hours. Always make fresh solutions.
4. Is 200 ppm safe for food contact surfaces?
Yes. According to safety standards, chlorine sanitizers under 200 ppm are safe when surfaces are rinsed properly.
Key Takeaways:
- 200 ppm = 200 mg solute per liter of solution
- For chlorine (5% bleach): use ~4 mL bleach per liter
- For hydrogen peroxide (3%): use ~6.7 mL per liter
- For fertilizers: calculate based on nutrient percentage
- Always verify ppm using meters or calculators
Making a 200 ppm solution is easy when you apply the right formula and tools.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
