Learn how to convert molarity to PPM step by step. Understand the formula, see worked examples, and discover common mistakes and shortcuts using online PPM calculators for chemistry, water testing, and hydroponics.
Understanding the Relationship Between Molarity and PPM
Chemists, lab technicians, and students often switch between molarity (mol/L) and PPM (parts per million) when describing concentration.
Both measure “how much solute is in a solution,” but in different ways:
- Molarity (M) = moles of solute / liter of solution
- PPM ≈ milligrams of solute / liter of solution
Because 1 mg/L ≈ 1 PPM for dilute aqueous solutions, converting is easy once you know the solute’s molar mass.
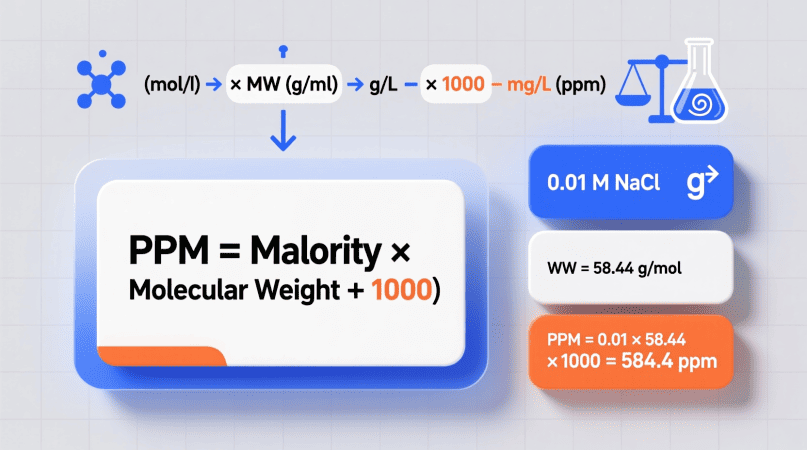
The Formula to Convert Molarity to PPM
PPM = Molarity × Molar Mass × 1000
Where:
- Molarity (M) = moles / liter
- Molar Mass (g/mol) = mass of 1 mole of solute
- The factor 1000 converts grams → milligrams
That’s it—multiply your molarity by the compound’s molar mass and by 1000 to get PPM.
For quick digital conversions, try our PPM to Molarity Calculator on the main site.
Example 1 – Convert NaCl Solution to PPM
Suppose you have a 0.001 M NaCl solution.
- Molar Mass = 58.44 g/mol
PPM = 0.001 × 58.44 × 1000 = 58.44 PPM
✅ Result: The solution contains ≈ 58 mg NaCl per liter of water.
If you want to cross-check, open the PPM to Grams Calculator—it verifies the same relationship in reverse.
Example 2 – Find PPM of a 0.005 M Calcium Chloride (CaCl₂) Solution
- Molar Mass = 110.98 g/mol
PPM = 0.005 × 110.98 × 1000 = 554.9 PPM
That means there are about 555 mg of CaCl₂ per liter—a common range in hydroponic nutrient mixes.
For nutrient-specific work, visit our Nutrient PPM Calculator.
Reverse Conversion – PPM to Molarity
If you ever need to find molarity from PPM, flip the equation:
Molarity = PPM / (Molar Mass × 1000)
Example: A 100 PPM NaCl solution → 100 / (58.44 × 1000) = 0.00171 M.
You can check your work with our PPM Solution Calculator.
Why Convert Molarity to PPM?
1. Consistency Across Fields
Environmental scientists and quality-control labs prefer PPM, while chemists prepare solutions by molarity.
Converting ensures both teams speak the same “language” of concentration.
2. Reporting Clarity
Water-testing results or fertilizer labels are almost always listed in PPM.
If you measure molarity in the lab, you’ll need this conversion before documentation.
See the PPM Standards for Drinking Water for benchmark values.
3. Practical Measurement
Field meters read TDS or EC in PPM, not molarity. When you mix or dilute solutions, converting lets you match what the meter displays.
Our EC to PPM Calculator helps bridge that gap.
Common Conversion Mistakes to Avoid
| Mistake | Why It Happens | Quick Fix |
|---|---|---|
| Using atomic mass instead of molecular mass | Missing combined weight of compound | Always use full molar mass (e.g., NaCl = 58.44 g/mol) |
| Forgetting × 1000 | Unit oversight | Remember: grams → milligrams |
| Assuming all solutions = water | Density affects precision | For non-aqueous solutions, multiply by density × 1000 |
| Rounded constants | Large errors in trace analysis | Keep 3–4 significant figures |
Need troubleshooting tips? Visit PPM Calculator Troubleshooting.
Real-World Applications ppm from molarity
Water Quality Analysis
Technicians convert molarity of dissolved salts or contaminants to PPM to compare against EPA limits.
Typical drinking water minerals range from 50–500 PPM.
Hydroponic and Soil Testing
Growers monitor nutrient molarity when mixing concentrates, then express the working solution in PPM.
Explore our PPM in Hydroponics Guide for ideal nutrient ranges.
Industrial and Manufacturing
In production settings, molarity-to-PPM conversions control cleaning-agent strengths and quality-defect rates, often measured in “defects per million.”
Quick Reference Table
| Molarity (M) | Molar Mass (g/mol) | Approx. PPM |
|---|---|---|
| 0.0001 | 58.44 (NaCl) | 5.84 |
| 0.001 | 58.44 (NaCl) | 58.4 |
| 0.01 | 58.44 (NaCl) | 584 |
| 0.001 | 18.02 (H₂O as solute) | 18.0 |
Bookmark this formula or use our homepage shortcut → PPM Calculator Home for instant results.
Pro Tip for Students and Researchers
When writing lab reports or theses:
- State both molarity and PPM for clarity.
- Include the molar mass source (from CRC or PubChem).
- Mention temperature if solutions deviate from 25 °C.
These details show precision and boost credibility in peer-reviewed work.
FAQ – Molarity and PPM Conversions
Is 1 mg/L always 1 PPM?
Yes for dilute water-based solutions; otherwise multiply by density.
Can I convert molality to PPM the same way?
Not exactly—molality uses mass of solvent instead of volume. You’ll need density data.
Why does Google show different answers for the same query?
Because some examples use approximations. Always verify molar mass and units manually or with a trusted calculator.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
