Learn how to calculate PPM from percentage using an easy formula, examples, and calculator tools. Understand the difference between percent and parts per million and when to use each in chemistry, water testing, and industry.
Understanding the Basics: What PPM and Percent Mean
Before jumping into calculations, let’s define the two units clearly.
- Percent (%) means parts per hundred. For example, 1% = 1 part solute per 100 parts of solution.
- PPM (parts per million) means parts per million. It’s used for much smaller concentrations — 1 part solute per 1,000,000 parts of solution.
Because both represent ratios, converting between them is simple — you just scale by a factor of 10,000.
🔹 The Formula to Convert Percentage to PPM
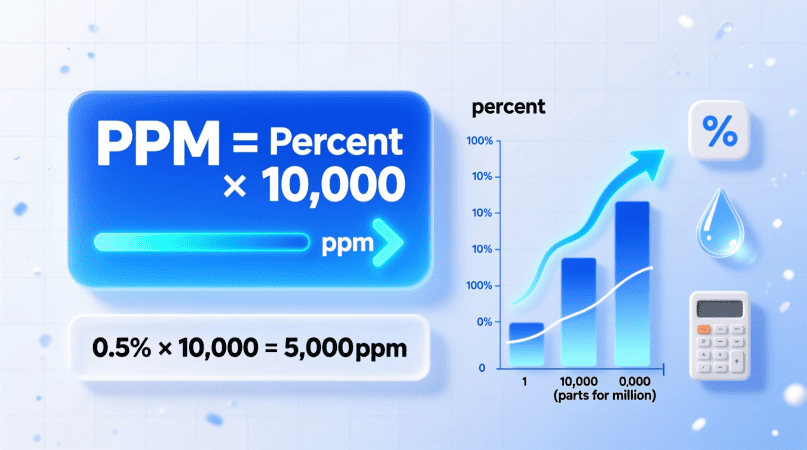
PPM = Percentage × 10,000
That’s all you need.
This works because:
- 1% = 1 part per 100
- 1 PPM = 1 part per 1,000,000
- So, 1,000,000 /100 = 10,000
Hence, multiplying a percent value by 10,000 gives you PPM.
To make this calculation instantly, try our PPM to Percent Calculator — it works both ways.
Example 1 – Convert 0.02% to PPM
PPM=0.02×10,000=200
✅ Result: 0.02% = 200 ppm
This means that for every million parts of the solution, 200 parts are solute.
🧮 Example 2 – Convert 3% to PPM
PPM=3×10,000=30,000
✅ Result: 3% = 30,000 ppm
This scale-up conversion is useful when describing stronger mixtures, like cleaning or disinfectant solutions.
For a practical example, check our Bleach PPM Calculator, which helps you compute exact concentrations for sanitizers.
🧮 Example 3 – Convert 0.0005% to PPM
PPM=0.0005×10,000=5
✅ Result: 0.0005% = 5 ppm
That’s a trace-level concentration, often seen in environmental monitoring or water testing.
If you work in water quality, also see PPM Standards for Drinking Water.
Reverse Formula: PPM to Percentage
Sometimes, you’ll need the opposite conversion:
Percentage = PPM ÷ 10,000
For instance, if your solution contains 250 ppm,
Percentage=250÷10,000=0.025%
You can verify this using our PPM to Percent Calculator or main PPM Calculator.
Why Converting Percentage to PPM Matters
Knowing how to switch between percentage and parts per million is vital in science, manufacturing, and agriculture. Here’s why it matters in different fields:
1. Laboratory Chemistry
Researchers often prepare stock solutions in percentages but need to report data in PPM, especially when dealing with trace concentrations.
If you also calculate concentrations using molarity, try our Molarity Calculator for related conversions.
2. Water and Environmental Testing
In water analysis, results like chlorine or fluoride levels are almost always expressed in PPM.
If your measurements are in percent, converting them ensures compliance with environmental standards.
See more in our Environmental Monitoring Using PPM.
3. Industrial and Food Manufacturing
Factories use percentages when mixing chemicals but report concentrations in PPM for safety documentation.
For example, sanitizer solutions labeled “0.05% chlorine” actually equal 500 ppm chlorine — a safe, effective level for cleaning surfaces.
4. Hydroponics and Agriculture
Growers track nutrient solutions in PPM for accuracy.
When nutrient manufacturers list “0.2% iron,” knowing that equals 2,000 ppm helps adjust feeding strength precisely.
You can explore this more in our PPM in Hydroponics Guide.
Quick Reference Conversion Table
| Percentage (%) | Equivalent PPM |
|---|---|
| 1.0 | 10,000 |
| 0.1 | 1,000 |
| 0.01 | 100 |
| 0.001 | 10 |
| 0.0001 | 1 |
Common Mistakes When Converting Percentage to PPM
- Forgetting to Multiply by 10,000
- Always multiply by 10,000 when converting % → PPM.
- Confusing PPM with PPB (parts per billion)
- 1% = 10,000 ppm but = 10,000,000 ppb.
- Use our PPM to PPB Calculator for deeper conversions.
- Rounding Too Early
- Keep at least three decimal places for accurate lab reports.
- Mixing Units (mass vs. volume)
- Always specify whether your percent is by weight (% w/w) or by volume (% v/v).
Pro Tip: Check Solution Density
The simple “×10,000” rule assumes water-like density (1 g/mL).
If your solution is denser, multiply the result by its density factor for better accuracy.
Example: If density = 1.2 g/mL, multiply final ppm by 1.2.
You can learn more about this in How PPM Is Measured in Labs.
When to Use Each Unit:
| Scenario | Use PPM | Use Percentage |
|---|---|---|
| Trace minerals in water | ✅ | ❌ |
| Fertilizer concentration | ✅ | ❌ |
| Industrial cleaning solution | ✅ | ✅ |
| Stock chemical mixtures | ❌ | ✅ |
When dealing with environmental safety, agriculture, or hydroponics, PPM gives you more precision at lower concentrations.
Frequently Asked Questions (FAQs)
1. Is 1% equal to 10,000 ppm?
Yes. 1% concentration equals 10,000 parts per million.
2. Does this formula work for gases or solids?
Yes, but for gases or solid mixtures, ensure units are consistent (mass/mass or volume/volume).
3. Can I convert ppm to mg/L using the same idea?
Exactly — in water-based solutions, 1 ppm ≈ 1 mg/L.
You can use our TDS to PPM Calculator to confirm this.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
