In laboratory science, PPM (parts per million) is a precise way to describe very small concentrations of a substance in a solution or mixture.
It literally means one part of solute per one million parts of solution. In water testing, for example, 1 PPM = 1 mg of solute per liter (mg/L).
Researchers, chemists, and technicians use PPM to track trace elements, verify purity, and ensure safety in everything from drinking water to industrial manufacturing.
Because small measurement errors can change outcomes, labs follow standardized procedures and calibrated tools to maintain consistency.
Common Laboratory Methods to Measure PPM
1. Gravimetric Analysis
This classical method involves weighing a substance before and after a reaction.
A reagent is added to form a solid precipitate. Once filtered, dried, and weighed, the mass reveals the concentration:
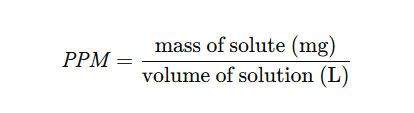
It’s reliable for detecting salts and metals but slower than digital techniques.
2. Spectrophotometry
Modern labs often use spectrophotometers to measure how much light a sample absorbs at a certain wavelength.
The higher the absorbance, the higher the concentration.
Using calibration curves, scientists convert absorbance to PPM values.
This method is ideal for testing chlorine, nitrate, and dissolved organic compounds in water.
For quick conversions, try the PPM to Molarity Calculator.
3. Titration
In a titration, a reagent of known concentration reacts with a sample until an endpoint is reached.
The volume of reagent used directly relates to the sample’s concentration in PPM.
It’s simple and effective for determining acidity, alkalinity, or chlorine levels, especially in field labs.
Learn more about related testing in How to Calculate PPM in Water.
4. Electrochemical Measurement
EC (Electrical Conductivity) meters and TDS (Total Dissolved Solids) meters measure ionic content in a solution.
The reading is automatically converted to PPM, depending on the calibration factor.
These instruments are common in environmental monitoring and hydroponic systems.
You can explore the EC to PPM Calculator to understand how conductivity relates to concentration.
Essential Lab Equipment
| Instrument | Purpose |
|---|---|
| Spectrophotometer | Measures light absorbance for colored compounds |
| Analytical balance | Provides high‑precision mass readings |
| pH/EC meters | Monitors acidity and conductivity |
| Titration burettes & pipettes | Enables accurate reagent delivery |
| Deionized water system | Prevents contamination in solutions |
Every instrument must be calibrated regularly using certified standards to ensure data reliability.
Example: Calculating PPM in a Lab Setting
Imagine you dissolve 5 mg of sodium chloride in 2 liters of distilled water:
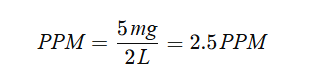
If you reduce the volume to 1 liter while keeping the solute amount constant, the PPM doubles to 5 PPM.
This relationship helps analysts adjust concentration precisely during solution preparation.
You can cross‑check results using the PPM Solution Calculator.
Best Practices for Accurate Results
Maintain Equipment Integrity
- Calibrate all meters weekly or before critical measurements.
- Use deionized or distilled water to avoid contamination.
- Keep reagents sealed and note expiration dates.
Control Environmental Conditions
- Perform measurements at a stable temperature (20–25 °C).
- Record pH and humidity if they could affect readings.
- Rinse glassware thoroughly before and after each test.
Verify and Document
- Include blank samples to detect contamination.
- Always compare results with a standard curve or certified reference.
- Document the method, instrument, and operator for traceability.
For troubleshooting guidance, see PPM Calculator Troubleshooting.
Real‑World Applications of PPM Measurement
Environmental Testing
Agencies test for heavy metals, nitrates, and chlorine residues in air, water, and soil to verify compliance with safety standards.
Accurate PPM measurement ensures that contaminants remain below legal thresholds.
Related reading: Environmental Monitoring Using PPM.
Water Quality and Pool Maintenance
Pool operators monitor chlorine and pH levels daily to prevent bacteria growth and equipment corrosion.
Check the Pool Chlorine PPM Calculator for quick adjustments.
Agriculture and Hydroponics
Farmers use PPM to manage nutrient concentration for optimal plant growth.
Too high causes root burn; too low leads to deficiency.
For growers, the Fertilizer PPM Calculator and Nutrient PPM Calculator are must‑have tools.
Conclusion
Measuring PPM in laboratories is a mix of precise calculation, instrument care, and chemical understanding.
Whether you’re testing water purity, analyzing fertilizer composition, or monitoring industrial emissions, accuracy in PPM measurement ensures safety, consistency, and credibility.
