If you’ve ever mixed a chemical solution or analyzed a sample, you’ve likely heard of PPM — short for parts per million. But what does it actually mean, and how do you calculate it correctly?
In chemistry, ppm is essential for expressing tiny concentrations of solutes in solutions, whether it’s salts in water, trace metals, or pollutants.
This guide explains how to calculate ppm in chemistry with clear examples, easy formulas, and links to calculators that help you avoid manual errors.
What Is PPM in Chemistry?
In simple terms, PPM (parts per million) measures how many parts of a substance exist in one million parts of a total solution or mixture.
It’s typically used when concentrations are too small to express as a percentage.
For aqueous (water-based) solutions:
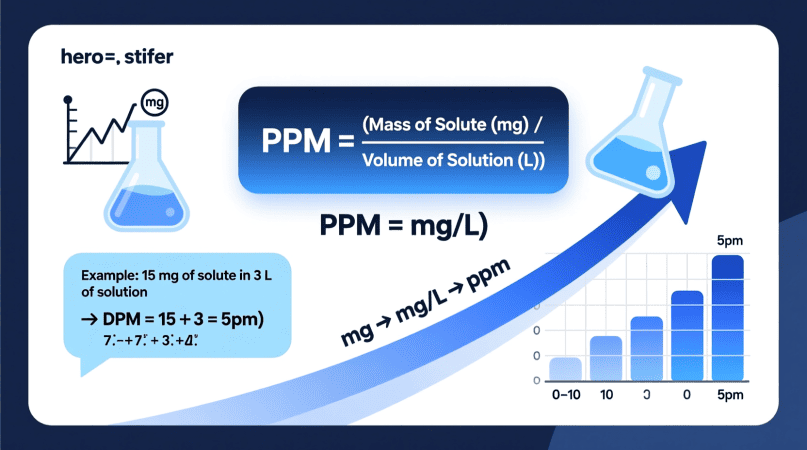
1ppm=1mg of solute per liter of solution (mg/L)
That means if you dissolve 1 milligram of a substance in 1 liter of water, you have a 1 ppm solution.
The Formula to Calculate PPM in Chemistry
The basic formula used in chemistry labs is:
PPM=Mass of Solute (mg) / Mass or Volume of Solution (L or kg)
For most water-based solutions, you can simplify this to:
PPM= Mg of Solute / L of Solution
This works because 1 liter of water weighs approximately 1 kilogram.
Step-by-Step Example: How to Calculate PPM
Let’s look at a simple example that every chemistry student or lab technician can relate to.
Example:
You dissolve 25 mg of sodium chloride (NaCl) into 2 liters of water.
What’s the ppm of the solution?
Step 1: Plug the values into the formula.
PPM = 25/2 = 12.5
✅ The concentration of the salt solution is 12.5 ppm.
If you want to skip manual calculations, use the PPM Solution Calculator — it applies the same formula and lets you switch between ppm, mg/L, and percent values instantly.
Converting PPM in Chemistry
Chemists often convert ppm into other concentration units depending on the experiment or instrument calibration.
1. PPM to Percent
Percent = PPM/10,000
Example:
100 ppm = 100 ÷ 10,000 = 0.01 %
You can check conversions like this with our PPM to Percent Calculator.
2. PPM to Molarity
To convert ppm to molarity, you need the substance’s molar mass:
Molarity = PPM / Molar Mass ×1000
This is useful when preparing solutions for titrations or chemical reactions.
Use our Molarity Calculator to get precise conversions automatically.
3. PPM to mg/L
In water solutions:
1ppm=1mg/L
This equivalence simplifies most environmental and analytical chemistry calculations.
PPM in Real Chemistry Applications
PPM is used everywhere — from lab analysis to industrial and environmental testing. Here are some common examples:
- Water Testing: Measuring dissolved minerals, hardness, or contaminants like iron and fluoride.
- Air Quality: Quantifying pollutant gases such as CO₂ or ozone.
- Food Chemistry: Checking trace additives or preservatives.
- Pharmaceuticals: Determining precise formulation concentrations.
- Agriculture: Managing nutrient levels in hydroponics and fertilizers.
For agricultural and hydroponic concentration control, you can use the Nutrient PPM Calculator.
Common Mistakes When Calculating PPM
Even small errors can affect accuracy in chemical analysis. Here are the most frequent ones:
- Wrong unit conversions – confusing mg with g or L with mL.
- Ignoring temperature effects – density changes with temperature can alter ppm readings slightly.
- Mixing up solution types – some equations apply to gases, others to liquids or solids.
- Rounding too early – always keep decimals until the final step.
To understand and fix these errors, check our PPM Calculation Mistakes article.
Example: PPM in Lab Water Sample
Suppose a chemist finds 0.003 g of copper (Cu) in a 2-liter water sample.
Convert grams to milligrams: 0.003 g = 3 mg.
Now apply the ppm formula:
PPM}= 3/2 = 1.5
So the copper concentration is 1.5 ppm.
For more detailed chemical conversions, use the tools available on PPM Calculator, which includes ppm-to-ppb, mg/L, and molarity functions.
FAQs About Calculating PPM in Chemistry
1. Why do chemists use ppm?
Because it’s an easy way to express trace concentrations without dealing with decimals or scientific notation.
2. Is 1 ppm the same as 1 mg/L?
Yes, in water-based solutions, 1 ppm equals 1 mg per liter.
3. How can I test ppm experimentally?
Use a colorimeter, spectrophotometer, or digital ppm meter to read solution concentration directly.
4. What’s a safe ppm level for drinking water?
That depends on the substance. For example, chlorine in water is safe below 4 ppm, while lead must be under 0.015 ppm.
Key Takeaways:
- Formula: ppm = mg solute ÷ L solution.
- 1 ppm = 1 mg/L in water-based chemistry.
- Use ppm for tiny concentrations where percentages are impractical.
- Convert easily between ppm, molarity, and percent using calculators.
- Verify results with test instruments or digital tools.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
