If you’re working in a lab, testing water quality, or preparing chemical standards, you might wonder:
How can I make a 1 ppm solution from a 100 ppm stock solution?
This process is called serial dilution — a precise method used to create lower concentrations from a higher one. In this guide, we’ll walk through the formula, calculations, and steps to make a 1 ppm solution from a 100 ppm solution safely and accurately.
What Does PPM Mean in a Solution?
PPM (parts per million) is a way to describe very small concentrations of a substance in a solution.
It tells you how many parts of solute exist per one million parts of the total mixture.
For water-based solutions:
1ppm=1mg of solute per liter of solution (mg/L)
So, a 100 ppm solution has 100 mg of solute per liter, while a 1 ppm solution has just 1 mg per liter.
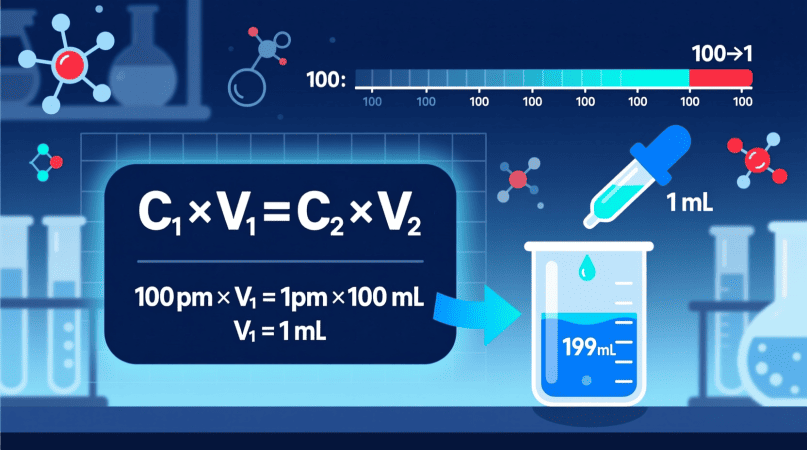
The Formula for Dilution (C₁V₁ = C₂V₂)
To make a lower concentration from a higher one, use the dilution formula:
C1×V1=C2×V2
Where:
- C₁ = initial concentration (ppm of the stock solution)
- V₁ = volume of stock solution to use (mL or L)
- C₂ = desired concentration (ppm of the final solution)
- V₂ = final total volume (mL or L)
This formula helps you calculate exactly how much of your 100 ppm stock you’ll need to make a 1 ppm solution.
Step-by-Step Example: Making 1 PPM from 100 PPM
Let’s say you want to prepare 100 mL (0.1 L) of a 1 ppm solution from a 100 ppm stock.
Step 1: Write down what you know
- (C₁ = 100) ppm
- (C₂ = 1) ppm
- (V₂ = 100) mL
Step 2: Plug into the formula
100×V1=1×100
V₁ = 100/100 = 1 mL
Step 3: Mix the solution
You’ll need 1 mL of the 100 ppm stock solution, then add 99 mL of distilled water to make the total volume 100 mL.
✅ That’s your 1 ppm solution.
To check your math or scale up larger batches, use our PPM Solution Calculator.
Why Dilution Accuracy Matters
Getting ppm concentrations right is critical in:
- Analytical chemistry (for calibration standards)
- Water testing (chlorine, iron, or fluoride levels)
- Agriculture and hydroponics (nutrient concentration control)
- Pharmaceutical labs (dose consistency)
Even a small miscalculation can throw off readings or harm test validity.
For example, using an overly concentrated sanitizer solution can damage equipment or affect results — something we cover in PPM Calculation Mistakes.
Scaling the Same Dilution for Any Volume
Once you understand the math, you can create any volume of a 1 ppm solution.
| Desired Volume | Formula Result | How to Prepare |
|---|---|---|
| 100 mL | (V₁ = 1) mL stock + 99 mL water | 1:100 dilution |
| 500 mL | (V₁ = 5) mL stock + 495 mL water | 1:100 dilution |
| 1 L (1000 mL) | (V₁ = 10) mL stock + 990 mL water | 1:100 dilution |
The ratio stays the same: 1 part of 100 ppm stock to 99 parts of water.
If you’re working with chlorine or peroxide, check your specific concentration using our Bleach PPM Calculator or Hydrogen Peroxide PPM Calculator.
Example: Making 1 PPM Chlorine Solution from 100 PPM
Let’s apply the formula to chlorine disinfection, which is common in food processing and water treatment.
If your stock chlorine solution is 100 ppm and you need 1 ppm for rinsing, the ratio is 1:100.
That means 1 liter of 100 ppm solution will make 100 liters of 1 ppm solution when diluted with clean water.
To calculate ppm for other concentrations (like 50 ppm or 200 ppm), try the Chemical Dosing PPM Calculator.
Making 1 PPM Solution in the Laboratory
When preparing ppm solutions in a lab, always follow these good practices:
- Use distilled or deionized water
Impurities in tap water can alter ppm readings. - Use clean glassware or volumetric flasks
Even trace residues can affect the concentration. - Label everything clearly
Include the concentration, chemical name, date, and preparer. - Verify with a meter or test kit
For chlorine, hydrogen peroxide, or nutrients, ppm meters and color tests help confirm results. - Mix gently
Avoid shaking too hard — air bubbles can affect final volume measurements.
Applications of 1 PPM Solutions
A 1 ppm standard is commonly used for:
- Calibrating measurement instruments (spectrophotometers, TDS meters)
- Trace element testing (iron, zinc, fluoride)
- Plant nutrient trials (hydroponic microelements)
- Checking sanitizer residue levels in rinse water
For more on ppm in agriculture and lab contexts, see our Nutrient PPM Calculator and PPM in Hydroponics Guide.
Safety and Accuracy Tips:
- Always add solute to water — never the reverse — to prevent splashes.
- Use pipettes or syringes for small volumes.
- If you’re unsure, double-check using a PPM Accuracy Calculator.
- Dispose of unused or expired solutions safely.
Proper measurement ensures consistency and avoids contamination or incorrect test results.
Key Takeaways:
- Goal: Make a 1 ppm solution from a 100 ppm stock.
- Formula: (C₁V₁ = C₂V₂)
- Example: 1 mL of 100 ppm stock + 99 mL water = 100 mL of 1 ppm solution.
- Maintain accuracy with proper tools and calculators.
- Always verify ppm using meters or testing kits.
Matthew is a chemical technology enthusiast and the creator of PPMCalculator.com — a platform dedicated to simplifying complex concentration and conversion formulas for students, researchers, and industry professionals. With years of experience exploring water chemistry, environmental monitoring, and laboratory analysis, he focuses on making scientific accuracy both practical and accessible.
When not writing or developing new tools, Shimul enjoys testing real-world chemical measurements, refining calculator algorithms, and helping learners understand the science behind PPM, EC, and TDS.
