PPM ⇄ PPMv Calculator
Convert between Parts Per Million by Mass (PPM) and Parts Per Million by Volume (PPMv)
PPMv = PPM × (Mair / Mcontaminant)
PPM = PPMv × (Mcontaminant / Mair)
Air Quality PPM to PPMv Calculator – Convert Concentration to PPMv
Quickly convert pollutant concentrations from PPM (mass) to PPMv (volume). Essential for environmental monitoring, EPA compliance, and air emissions analysis.
Welcome to the world of gas concentration! Whether you’re a student, an environmental scientist, or just someone curious about what’s in the air around you, understanding the difference between PPM and PPMv is crucial. This guide will walk you through how to use the PPM to PPMv calculator and explain the science behind it in simple terms.
What’s the Difference Between PPM and PPMv?
At its core, PPM stands for “parts per million.” It’s a way of expressing a very small concentration. For example, if you have 1 PPM of a substance in a million parts of a solution, it means that for every million particles, one of them is the substance you are measuring.
However, things get a bit more complicated with gases. Gas concentrations can be measured in two primary ways: by mass or by volume.
- PPM (by mass or weight): This is often used for liquids or solids and represents the mass of a substance per million parts of the total mass. For example, a water sample with 1 PPM of lead means there is 1 milligram of lead for every kilogram of water.
- PPMv (parts per million by volume): This is the standard for measuring gas concentrations. It represents the volume of a substance per million parts of the total volume. This is the more accurate way to describe how many molecules of a specific gas are present in the air. For instance, a CO2 concentration of 420 PPMv means that for every million gas molecules in a given volume of air, 420 of them are carbon dioxide.
The relationship between PPM and PPMv is not a simple one-to-one conversion because it’s highly dependent on environmental conditions like temperature and pressure. That’s where our calculator becomes incredibly useful.
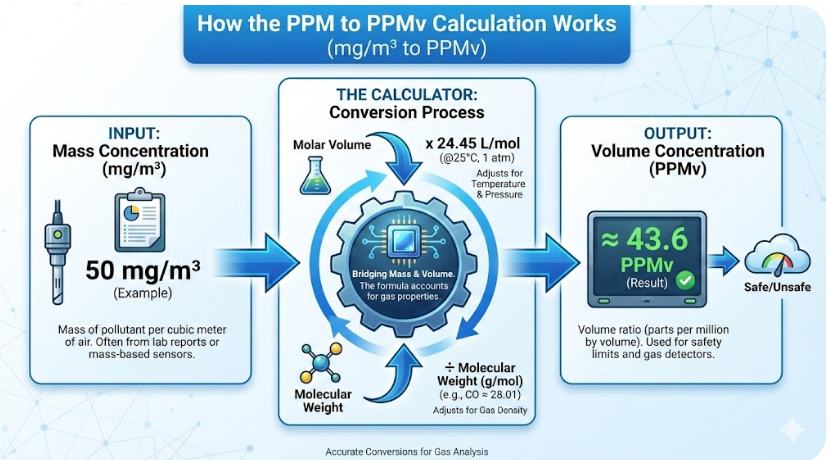
How to Use the PPM to PPMV Calculator
Using the PPM to PPMv calculator is straightforward, but it’s important to understand what each input field represents.
Step 1: Enter Your PPM Value
The first field asks for the “PPM (Parts Per Million)” value. This is the concentration you already have, likely from a sensor or data sheet. Enter the numerical value here.
Step 2: Input Temperature
Temperature is a critical variable in this conversion. Gases expand and contract with changes in temperature, which directly affects their concentration by volume. The calculator provides options for both Celsius (°C) and Fahrenheit (°F). Select the correct unit and enter the ambient temperature at which your PPM measurement was taken.
Step 3: Input Pressure
Similarly, pressure affects the volume of a gas. A higher pressure compresses the gas, increasing its concentration by volume. The calculator supports several common pressure units:
- kPa (kilopascals): The standard SI unit of pressure.
- atm (atmospheres): Standard atmospheric pressure at sea level.
- psi (pounds per square inch): A common unit in the United States.Select the unit that matches your data and enter the pressure value.
Step 4: Click “Calculate PPMv”
Once you have entered all the required values, simply click the “Calculate PPMv” button. The calculator will perform the conversion and display the result in a clean, easy-to-read format.
This tool is part of PPMCalculator.com — a collection of simple, reliable calculators designed to help students, professionals, and hobbyists perform accurate PPM, dilution, and concentration calculations with ease.
The Science: Formula & Conversion Explanation
The conversion from PPM to PPMv is rooted in the Ideal Gas Law, a fundamental principle of chemistry and physics. The Ideal Gas Law, often expressed as PV=nRT, describes the relationship between pressure, volume, temperature, and the number of moles of a gas.
For our purposes, the key insight is that the volume of a gas is directly proportional to its temperature (in Kelvin) and inversely proportional to its pressure. The calculator uses a simplified version of this principle to convert your PPM value (which is a mass-based measurement in this context) to a volume-based one.
The formula used by the calculator is:
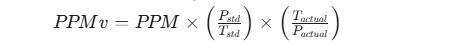
Where:
- PPMv is the final parts per million by volume.
- PPM is the initial parts per million (by mass).
- Pstd and Tstd are standard pressure and temperature. The calculator uses STP (Standard Temperature and Pressure) where temperature is 0°C or 273.15K and pressure is 1 atm.
- Pactual and Tactual are the actual measured pressure and temperature, converted to standard units (atm and Kelvin, respectively).
By accounting for these variables, the calculator provides a much more accurate and scientifically grounded PPMv value.
Applications & Use Cases
The ability to accurately convert between PPM and PPMv is vital in several industries and scientific fields.
Environmental Monitoring
Environmental scientists and engineers rely on this conversion to monitor air quality. For example, regulatory agencies often set limits for pollutants like carbon monoxide or ozone in PPMv, so converting raw sensor data is a necessary step to ensure compliance.
Industrial Safety
In industrial settings, gas detectors are essential for worker safety. Many sensors measure gas concentration in PPM (by weight), but safety standards and permissible exposure limits are often defined in PPMv. An accurate conversion is critical to ensure that employees are not exposed to dangerous levels of toxic or flammable gases.
Climate and Atmospheric Science
Researchers studying climate change and atmospheric composition use PPMv to measure greenhouse gas concentrations. Data from CO2 sensors, for instance, is often converted to PPMv to be consistent with international reporting standards and historical records.
Medical and Healthcare
In medical applications, such as respiratory gas analysis or anesthesia monitoring, gas concentrations are often measured in PPMv. Accurate readings are vital for patient safety and diagnostics.
Related Calculators and Resources:
- Agriculture and Fertilizer PPM Calculations
- Environmental Monitoring Using PPM
- PPM Glossary
- PPM vs TDS vs EC
- How to Use a PPM Calculator for Accurate Measurement
- Dosage Calculation for PPM
- Molarity and PPM
FAQs Section
Q1: Why do I need to convert PPM to PPMv?
A1: PPM is a general measure that can refer to mass or volume, but for gases, the volume-based concentration (PPMv) is the standard. This conversion is necessary because gas volume changes with temperature and pressure, which means a PPM measurement by weight can be misleading without correcting for these environmental factors. Converting to PPMv provides a true and comparable measure of gas molecules in the air.
Q2: What are “standard conditions” for this conversion?
A2: “Standard conditions” or STP (Standard Temperature and Pressure) are a set of widely accepted reference points for scientific calculations. The most common standard is 0°C (273.15K) and 1 atm of pressure. Using these fixed points allows for consistent and comparable calculations regardless of where the data was collected.
Q3: Can I use this calculator for liquids or solids?
A3: No, this calculator is specifically designed for gas conversions, as the underlying formula is based on the behavior of gases. For liquids or solids, PPM is a mass-per-mass measurement and doesn’t require a volume-based conversion.
Q4: What happens if my temperature or pressure is very different from standard conditions?
A4: The conversion becomes even more important! The greater the difference between your ambient conditions and standard conditions, the larger the discrepancy will be between your initial PPM value and the final PPMv value. The calculator accurately accounts for these differences to provide a precise result.
Q5: Is this conversion applicable to all gases?
A5: The Ideal Gas Law is a good approximation for most gases under typical atmospheric conditions. While there are minor deviations for real gases, this calculator provides a highly accurate conversion for most practical applications in environmental monitoring, industrial safety, and general science.
Q6: Why is PPM used in the first place if PPMv is the standard?
A6: Many sensors and instruments measure gas concentration based on the mass of the substance that reacts with the sensor. It is often easier to measure mass directly, which yields a PPM (by mass) value. The conversion to PPMv is then done computationally to align with a volume-based standard for reporting and comparison.
Q7: What’s the relationship between PPMv and percentage (%)?
A7: A percentage is parts per hundred. Since a million is 10,000 times a hundred, you can easily convert PPMv to percentage by dividing by 10,000. For example, 10,000 PPMv is equal to 1%.
